#Cellular Heterogeneity
Explore tagged Tumblr posts
Text
Reference saved in our archive
The analysis identified numerous significant pathways, including Pathways in Parkinson’s disease, Prion diseases and COVID-19. ViBe identified a wide range of biological process, cellular component and molecular function associated with tumorigenesis. Furthermore, we validate that the SPP1 signaling pathway is essential for cell–cell crosstalk, specifically functioning as a positive feedback loop between cancer-associated fibroblasts and macrophages.
"Just a cold"
6 notes
·
View notes
Text
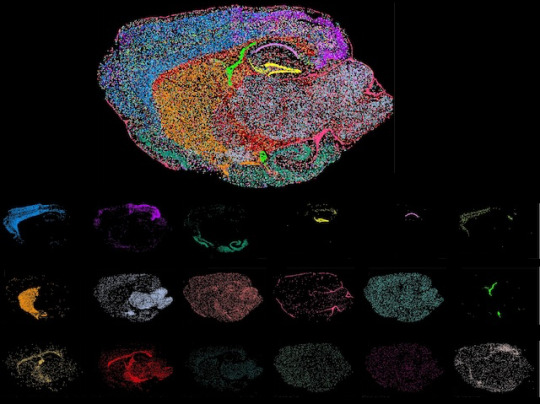
All Together Now
Profiling single cells within tissues according to the activity of multiple genes using methods called Fluorescence In Situ Hybridization of Cellular HeterogeneIty and gene expression Programs (FISHnCHIPs) enables mapping of cell types within a tissue while preserving its architecture. Different cell types in a mouse whole brain section are shown in the image individually and as a composite
Read the published research article here
Adapted image from work by Xinrui Zhou and Wan Yi Seow, and colleagues
Genome Institute of Singapore, Agency for Science, Technology and Research (A*STAR), Singapore, Singapore
Image originally published with a Creative Commons Attribution 4.0 International (CC BY 4.0)
Published in Nature Communications, March 2024
You can also follow BPoD on Instagram, Twitter and Facebook
12 notes
·
View notes
Text
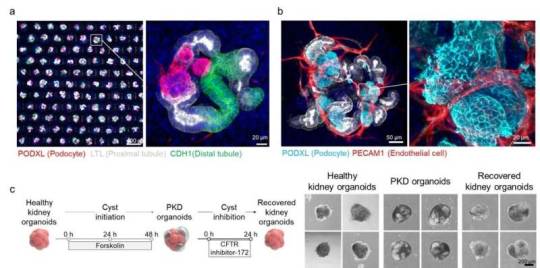
Scalable production of high-quality organoids: Innovative platform utilizes 3D engineered nanofiber membrane
A research team has successfully developed a platform capable of scalable, uniform production of organoids that mimic biological functions. Their research has recently been published in the journal Nature Communications. Organoids are three-dimensional cellular constructs that replicate the functions of human organs, attracting significant attention in the fields of human organ development, disease modeling, and regenerative medicine research. However, the heterogeneity and low reproducibility of organoids present challenges to their scalable production, limiting their practical application in clinical trials and drug development processes. Additionally, current technologies face limitations in producing organoids at scale, falling short of meeting industrial demands.
Read more.
#Materials Science#Science#Nanofibers#Membranes#Medical technology#Tissue engineering#Nanotechnology#POSTECH
6 notes
·
View notes
Note
What's your favourite phospholipid?
This question is basically impossible to answer. Not because of my usual take on favorites questions, which is "I can't pick", but because phospholipids tend to form heterogenous layers composed of extremely similar phospholipids that differ slightly to alter the properties of the structure they're making.
For example, the cell membrane will alter its lipid membrane composition to make the membrane more or less rigid. A longer fatty acid chain means more ability for hydrophobic bonds to form with its neighbors, and therefore, a membrane is more rigid if it's composed of more phospholipids with longer fatty acid chains. Membrane fluidity or rigidity is dependent on the role of the cell (eg, is it sessile or motile?) As well as conditions such as temperature (which, tbf is fairly constant in mammals but still has fluctuations on the Cellular level and suffers between the core and extremities). Eg, more rigid membranes are better to counteract the added lipid fluidity at higher temperatures, and vice versa.
And maybe I'm a dumbass, but I'm pretty fucking sure that every single fucking length of phospholipid possible in cell membranes has its own fucking name. And no, I'm not gonna choose between the 10 or so options that are possible, okay? I can't even remember how many there are exactly.
So this question is damn near impossible to answer, because of course I'm gonna choose membrane phospholipids. But getting more specific than that is basically impossible lmao
13 notes
·
View notes
Text
FinFET Technology Market Size Elevating Semiconductor Performance to New Heights
The FinFET Technology Market Size marks a significant milestone in semiconductor fabrication, offering higher performance, lower power consumption, and enhanced scalability compared to traditional planar transistors. With devices shrinking to sub-10 nm nodes, the introduction of FinFETs—transistors featuring a 3D fin structure—has become pivotal for sustaining Moore's Law. These advancements are redefining computing, cellular, automotive, and edge AI applications.
According to Market Size Research Future, global FinFET technology is projected to grow significantly by 2030, driven by increasing demand for high-performance ASICs, 5G infrastructure, and AI accelerators. The shift toward smaller process nodes and energy-efficient designs continues to fuel investment across foundries and OEMs.
Overview of FinFET Technology
FinFET (Fin Field-Effect Transistor) represents a 3D transistor family designed for low leakage and efficient switching at nanometer-scale nodes. By wrapping a thin silicon fin around the gate, FinFETs improve electrostatic control and reduce short-channel effects—critical for modern chip performance.
You'll find FinFETs at the core of system-on-chip (SoC) architectures powering smartphones, high-speed computing, networking hardware, and high-throughput mobile devices. Applications span from Qualcomm's Snapdragon processors to Nvidia’s Ampere GPUs, as both industries prioritize performance-per-watt and thermal limits.
Key Growth Drivers
Advanced Process Node Demand The continuous scaling to 7 nm, 5 nm, and beyond requires FinFETs to maintain channel control, reduce leakage, and boost transistor density.
5G and Networking Infrastructure Infrastructure equipment—like RF front-end modules and baseband SoCs—rely on FinFETs to support high frequencies and low power under elevated thermal loads.
AI Accelerators & Edge Compute FinFETs offer the transistor scaling needed to maintain performance gains in data centers and energy-efficient edge AI processors.
Automotive and Industrial Electronics With automotive electronics requiring high reliability and low power, FinFET integration in ADAS, radar, and EV power components is rising sharply.
Market Size Segmentation
By Node Range: 14 nm & below / 10 nm–7 nm / 5 nm and below
By Transistor Type: High Performance (HP), Low Leakage (LO), High Voltage (HV)
By End‑User: Smartphones, Data Centers, 5G Infrastructure, Automotive, Defense & Aerospace, Consumer Electronics, Industrial IoT
Regional Dynamics
Asia-Pacific leads due to TSMC, Samsung, and Chinese foundries aggressively ramping advanced nodes.
North America excels in design leadership through companies like Intel and Nvidia, including advanced integration of FinFETs in HPC and AI chips.
Europe focuses on automotive-grade FinFETs, with contributions from Infineon and STMicroelectronics in the energy and mobility sectors.
Key Industry Players
TSMC – Dominant pure-play foundry with leadership in high-volume 5 nm and 3 nm FinFET production.
Samsung Foundry – Offers advanced FinFET nodes and innovative packaging through SoC integration.
Intel – Transitioning to FinFET and gate-all-around architectures in its IDM 2.0 strategy.
GlobalFoundries, UMC, and SMIC – Deliver mature FinFET nodes for automotive, industrial, and mid-range SoCs.
Cadence, Synopsys, Mentor (Siemens) – Provide EDA tools for FinFET-based design, modeling, and verification.
ARM, Qualcomm, Broadcom, Nvidia – Utilize FinFET technologies in their SoC portfolio.
Emerging Trends
Gate-All-Around (GAA) Evolution – Next-gen FETs transition from FinFET towards GAA structures (e.g., nanosheet), extending device scaling.
2.5D/3D Chiplets & Heterogeneous Packaging – Integrating FinFET chips via advanced interconnects for modular, multi-die systems.
Beyond FinFET – Research into nanosheet, nanowire transistors, and monolithic 3D stacking is progressing rapidly to sustain scaling.
AI/ML for Process Control – real-time analytics optimize yields and lower defects in FinFET production.
Challenges Ahead
Rising manufacturing costs at sub-5 nm nodes, requiring ROI over large orders.
Thermal management and interconnect resistance, demanding advanced packaging materials and cooling solutions.
Design complexity, requiring sophisticated EDA tools and IP for timing and variability control.
Geopolitical constraints and supply chain uncertainties necessitating diversification and capacity investment.
Future Outlook
FinFET technology will remain dominant through the 2025–2028 horizon, enabling cutting-edge applications in AI, 5G, and edge computing. The transition to GAA and further miniaturization promises continued performance gains. With increasing investment in specialized FinFET fabs and design platforms, this evolution supports the growing demands of intelligent, connected devices.
Related Insights
Explore adjacent technology fields shaping chip innovation:
Radar Lidar Technology for Railway Applications Market Size
Quadruped Robot Market Size
Oxygen Gas Sensor Market Size
E‑Tailing Solution Market Size
Consumer Electronics Mini LED Market Size
D‑Shaped Connector Market Size
Debris Extraction Tool Market Size
Diaper Attachment Sensor Market Size
Digital Holographic Display Market Size
Digital Photo Printing Market Size
Discrete Graphics Microprocessor and GPU Market Size
Diving Compressor Market Size
Explosion Proof Mobile Communication Device Market Size
0 notes
Text
Revolutionary Bispecific Antibodies: The Next Frontier in NSCLC Therapy

Cancer immunotherapy is experiencing a paradigm shift with the development of next-generation bispecific antibodies specifically designed for Non-Small Cell Lung Cancer (NSCLC) treatment. These sophisticated therapeutic agents represent a quantum leap from traditional monoclonal antibodies, offering dual-targeting capabilities that address the complex molecular landscape of lung cancer.
The Science Behind Dual-Target Therapy
Bispecific antibodies function as molecular bridges, simultaneously engaging two distinct cellular targets to create synergistic therapeutic effects. Unlike conventional single-target approaches, these advanced molecules can simultaneously modulate immune checkpoint pathways while disrupting tumor growth signals. This dual mechanism addresses the inherent complexity of NSCLC, where multiple pathways contribute to disease progression and treatment resistance.
The innovative design of bispecific antibodies allows clinicians to overcome the limitations traditionally associated with monotherapy approaches. By targeting complementary pathways simultaneously, these agents potentially reduce the evolutionary pressure that leads to treatment resistance, a common challenge in advanced NSCLC management.
Breakthrough Developments in Checkpoint Modulation
Volrustomig exemplifies the cutting-edge approach to dual checkpoint inhibition. This innovative bispecific antibody simultaneously targets PD-1 and TIGIT pathways, two crucial immune checkpoints that tumors exploit to suppress anti-cancer immunity. The strategic combination addresses multiple layers of immune evasion mechanisms that NSCLC cells employ.
Clinical investigations have revealed promising efficacy signals in patients with treatment-refractory disease. The dual targeting strategy appears particularly beneficial for patients who have developed resistance to conventional PD-1 inhibitors, offering renewed therapeutic opportunities where traditional approaches have failed.
Vascular and Immune System Integration
The BioNTech PD 1 VEGF bispecific platform represents a sophisticated approach combining immunotherapy with anti-angiogenic therapy. This dual-targeting strategy addresses both immune checkpoint inhibition and tumor vascularization, two fundamental processes that support cancer progression and metastasis.
The scientific rationale centers on the interconnected relationship between tumor angiogenesis and immune suppression. VEGF-driven blood vessel formation creates an immunosuppressive microenvironment that limits immune cell infiltration. By simultaneously blocking these pathways, this bispecific approach aims to normalize tumor vasculature while enhancing immune system activation.
Expanding Therapeutic Horizons
The non-small cell lung cancer pipeline now includes multiple bispecific platforms targeting diverse molecular combinations. Emerging agents are exploring HER family receptor combinations, metabolic pathway modulators, and T-cell redirecting mechanisms. Each approach addresses specific molecular subtypes and resistance patterns observed in NSCLC.
Novel combinations under investigation include CD3-engaging bispecifics that physically redirect cytotoxic T-cells to tumor sites, and agents targeting growth factor receptor networks that drive tumor proliferation. These diverse approaches reflect the growing understanding of NSCLC molecular heterogeneity and the need for precision medicine strategies.
Manufacturing and Delivery Innovations
The complexity of bispecific antibodies presents unique challenges in pharmaceutical development. Advanced protein engineering techniques are being employed to optimize stability, reduce immunogenicity, and enhance tissue penetration. Manufacturing processes have evolved to accommodate the sophisticated molecular architecture required for dual-targeting functionality.
Delivery optimization involves careful consideration of dosing schedules, combination protocols, and patient selection criteria. Clinical teams are developing biomarker-guided approaches to identify patients most likely to benefit from specific bispecific platforms.
Clinical Translation and Patient Impact
Current clinical trials are systematically evaluating bispecific antibodies across different NSCLC stages and molecular subtypes. Phase I and II studies are establishing safety profiles while identifying optimal dosing regimens. Combination strategies with existing therapies are being explored to maximize therapeutic synergy.
Patient selection strategies are evolving based on tumor molecular profiling, immune system status, and previous treatment history. Predictive biomarkers are being identified to guide treatment decisions and optimize clinical outcomes.
Addressing Treatment Resistance Mechanisms
Bispecific antibodies offer unique advantages in overcoming acquired resistance to standard therapies. By engaging multiple pathways simultaneously, these agents can circumvent single-pathway resistance mechanisms that limit conventional treatments. This approach is particularly valuable for patients with progressive disease following standard-of-care interventions.
The multi-target strategy addresses both intrinsic and acquired resistance patterns, potentially extending treatment durability and improving long-term outcomes for patients with advanced NSCLC.
Future Directions and Clinical Integration
The integration of bispecific antibodies into standard NSCLC care protocols represents a significant advancement in precision oncology. As clinical data matures, these innovative therapies are expected to become cornerstone treatments for specific patient populations.
Ongoing research focuses on optimizing combination strategies, identifying predictive biomarkers, and developing next-generation bispecific platforms with enhanced efficacy profiles. The continued evolution of this therapeutic class promises to further transform NSCLC treatment paradigms and improve patient outcomes.
Latest Blogs Offered By DelveInsight:
An Ageing Population and Upcoming Therapies: What is shaping the Alzheimer’s Disease Market Scenario?
Top 10 Most Promising Drugs In The Amyotrophic Lateral Sclerosis Treatment Landscape
Amyotrophic Lateral Sclerosis Treatment: The Journey of Radicava and Highlights of Riluzole Formulations
How Will Emerging Therapies Drift the Amyotrophic Lateral Sclerosis (ALS) Treatment Landscape?
Unveiling Amyotrophic Lateral Sclerosis (ALS) Treatment Frontiers: Navigating Challenges, Overcoming Setbacks, and Emerging Therapeutic Horizons
Pompe Disease Market
Latest Reports:-
Dry Amd Market | Severe Dry Eye Market | Duodenoscope Market | Dysmenorrhea Market | Echocardiography Devices Market | Electrophysiology Devices Market | Global Electrophysiology Devices Market | Eoe Market | Eosinophilia Market | Eosinophilic Gastroenteritis Market | Erectile Dysfunction Devices Market | Erectile Dysfunction Market | Ewing Sarcoma Market | Exocrine Pancreatic Insufficiency Market | Fabry Disease Market | Familial Amyloid Polyneuropathy Market | Fatty Acid Oxidation Disorders Market | Febrile Neutropenia Market | Fecal Incontinence Market | Female Infertility Market | Fetal And Neonatal Monitoring Devices Market Market | Fibrodysplasia Ossificans Progressiva Market | Focal Segmental Glomerulosclerosis Market | Follicular Lymphoma Market | Foot And Ankle Devices Market | Fragile X Syndrome Market | Fuchs Dystrophy Market | Functional Dyspepsia Market | Dyspepsia Market | Gastroesophageal Junction Adenocarcinoma Market | Gastroparesis Market | Gaucher Disease Market | Gene Therapy For Ocular Rare Disease Market | Gene Therapy Market | Germ Cell Tumor Market | Gingivitis Market | Glaucoma Market | Primary Open-angle Glaucoma Market | Global Kinase Inhibitor Market
#biontech pd 1 vegf bispecific#volrustomig mechanism of action#Bispecific Antibodies#Non-small cell lung cancer#Non-Small Cell Lung Cancer (NSCLC)#Non-small cell lung cancer Pipeline#non-small cell lung cancer treatment#NSCLC#NSCLC treatment
0 notes
Text

Single-Cell Analysis Award
!! Computational Biologists Awards !!!
🏆Single-Cell Analysis Award– Nominate Now! 🚀
📅 30-31 May 2025
📍 Paris, France
single-cell biology, celebrating breakthroughs that enhance our understanding of cellular heterogeneity, disease mechanisms, and precision medicine.
🔹 Nominate Now!
0 notes
Text
Spatial Genomics Transcriptomics Market to Surge with Single-Cell Resolution

The Global Spatial Genomics Transcriptomics Market is estimated to be valued at USD 335.8 Mn in 2025 and is expected to exhibit a CAGR of 13% over the forecast period 2025 to 2032.
Spatial genomics and transcriptomics products enable high-resolution mapping of gene expression within tissue sections, combining imaging-based sequencing with computational analytics. These platforms offer single-cell resolution and multiplexed detection of thousands of transcripts, giving researchers unprecedented insights into cellular heterogeneity and microenvironmental interactions. Advantages include precise localization of biomarkers, improved target validation in oncology and neuroscience, and accelerated drug discovery through deeper tumor-immune profiling. As demand grows for integrated multi-omics and digital pathology workflows, Spatial Genomics Transcriptomics Market Insights is an instruments and reagents—such as barcoded slides, fluorescence-based probes, and advanced bioinformatics software—are becoming essential tools in academic and commercial research labs. The need for robust spatial profiling in personalized medicine and biomarker discovery continues to drive adoption, while ongoing innovations reduce data-analysis bottlenecks.
Get more insights on,Spatial Genomics Transcriptomics Market
#CoherentMarketInsights#SpatialGenomicsTranscriptomicsMarket#SpatialGenomicsTranscriptomics#SpatialGenomicsTranscriptomicsMarketInsights#Kits&Reagents
0 notes
Text
MediaTek Kompanio 900T For Enhanced Mobile Computing

Kompanio900T MediaTek
MediaTek Kompanio 900T chips for high-performance mobile computing platforms seek capability, connectivity, and battery life. The octa-core CPU has two powerful Arm Cortex-A78 cores and six Cortex-A55 cores for performance and responsiveness and is constructed on the power-efficient TSMC N6 (6nm-class) manufacturing process. The processor offers versatile system designs with LPDDR4X or LPDDR5 memory and UFS 2.1 or UFS 3.1 storage and can power 2K (2000×1200) screens at 120Hz.
Full 2G-5G Sub-6GHz connection, 5G Carrier Aggregation, True Dual 5G SIM (SA+SA), and MediaTek 5G UltraSave power efficiency are features of its integrated, certified 5G modem. Bluetooth 5.2 and Twin Antenna Wi-Fi 6 (802.11ax) with 2×2 MIMO boost connectivity. The processor supports 108MP cameras with an Arm Mali-G68 MC4 GPU, MiraVision HDR, and AI Accelerator.
Key MediaTek Kompanio 900T features include
The TSMC N6 (6nm-class) chip production process is up to 8% more efficient than the N7 (7nm-class) technology, making the chip power-efficient. This technology lets device manufacturers create mobile computer systems with unsurpassed battery life.
Processor: Its octa-core CPU is equipped with six Arm Cortex-A55 processors and two potent Arm Cortex-A78 processors. Increased gaming frame rates, multitasking, speedier applications, and a more responsive system perfect for pen use are all made possible by these Cortex-A78 processors. The 64-bit CPU supports heterogeneous multiprocessing.
Memory and Storage: The choice between LPDDR5 or LPDDR4X memory and UFS 3.1 or UFS 2.2 storage is available to device manufacturers. As a result, designs may be adjusted to fit price points and market performance expectations.
Display Support: With a maximum resolution of 2K (2000×1200), the chip can work with fast 120Hz monitors. High refresh rates are said to provide a discernible improvement in the user experience, enhancing zero-blur gaming, facilitating smoother web scrolling, and enabling faster pen and touch input response times.
Connectivity5G: Along with complete 2G-5G cellular connection, including all 5G Sub-6GHz frequency bands, it comes with an integrated, certified 5G modem. It supports 5G Carrier Aggregation (CA), SA and NSA modes, and 5G-CA Mixed Duplex TDD+FDD for improved layer coverage and quicker average speeds. The chip provides True Dual 5G SIM (5G SA + 5G SA) connectivity, which enables quality voice and video conversations (VoNR) on both connections for a more reliable 5G experience.
5G UltraSave: They include MediaTek 5G UltraSave power-saving technologies, which are said to save up to 50% more energy in 5G connected settings than certain alternatives. These technologies may be used in both standalone and non-standalone networks. Wi-Fi: Up to 66% faster speeds than the previous generation are claimed with the integration of Twin Antenna Wi-Fi 6 (802.11ax) with 2×2 MIMO.
Other Wireless: Additionally, GNSS (which supports systems like GPS, BeiDou, Glonass, and Galileo) and Bluetooth 5.2 are included.
Graphics and Video: A GPU called Arm Mali-G68 MC4 is included in the chip. Numerous video encode and decode standards, including H.264, H.265/HEVC, MPEG-1/2/4, VP-9, and AV1, are supported.
MiraVision: A set of technologies called MiraVision HDR Video Playback & Display is part of it; it automatically modifies the video stream parameters. Improved HDR10+ playback with per-frame local tone mapping, real-time SDR to HDR conversion, and improving HDR10 video to seem like HDR10+ are some of the main improvements highlighted.
AI: There is an AI Accelerator available.
Camera Support: Camera options up to 108MP single camera or twin 20MP+20MP setups are supported by the MediaTek Kompanio 900T. It has hardware capabilities including HDR video, 3X HDR-ISP, MFNR, 3DNR, AINR, and hardware depth and warping engines. It can record video at a maximum resolution of 3840×2160.
#MediaTekKompanio900T#LPDDR5memory#MiraVision#MediaTek#AIAccelerator#Kompanio#LPDDR4Xmemory#News#Technews#Technology#Technologynews#Technologytrends#govindhtech
1 note
·
View note
Text
Cell Separation: Techniques Used to Isolate Specific Cell Types From Complex Biological Samples
All living organisms are composed of cells, the fundamental structural and functional units of life. Within multicellular organisms, cells perform highly specialized functions and combine to form tissues and organs through interactions and transport of molecules. Being able to isolate specific cell types is essential for understanding cellular function in both health and disease. Cell separation refers to techniques used to separate a targeted cell population from a heterogeneous cell mixture based on unique physical or biochemical properties. These purified cell preparations are then available for characterization, analysis and downstream applications like cell culture, tissue engineering and regenerative medicine. Cell Separation - https://www.patreon.com/posts/cell-separation-128189388
0 notes
Text
Spatial Omics Market: Future Trends and Market Potential 2024-2032
The Spatial Omics Market was valued at USD 355.8 million in 2022 and is projected to reach USD 847.6 million by 2030, growing at a CAGR of 11.5% during the forecast period 2023–2030. This impressive growth trajectory highlights the increasing adoption of spatial omics technologies in biomedical research and clinical diagnostics, particularly in fields such as oncology, neuroscience, and immunology.
Market Overview Spatial omics technologies combine advanced imaging and molecular profiling tools to map biological molecules within their spatial context in tissues. These techniques are becoming increasingly crucial in understanding complex disease mechanisms at a cellular level, aiding in the development of precision medicine and targeted therapies. The integration of spatial transcriptomics, spatial proteomics, and metabolomics is reshaping the way researchers analyze tissue architecture and cellular heterogeneity.
Get Free Sample Report @ https://www.snsinsider.com/sample-request/4257
Regional Analysis
North America currently dominates the spatial omics market due to robust R&D infrastructure, rising healthcare investments, and strong presence of biotech companies and research institutes.
Europe follows closely, supported by increased government funding for genomics and healthcare innovation.
Asia-Pacific is expected to witness the fastest growth, fueled by expanding healthcare systems, growing genomic research interest, and collaborations across academic and commercial sectors.
Latin America and Middle East & Africa are emerging markets showing promise due to rising diagnostic needs and improving healthcare access.
Market Segmentation
By Technology
Spatial Transcriptomics
Spatial Proteomics
Spatial Metabolomics
Multiplexed Ion Beam Imaging
By Product
Instruments
Consumables
Software
By Application
Oncology
Neurology
Immunology
Drug Discovery
Others
By End-User
Academic & Research Institutions
Pharmaceutical & Biotechnology Companies
Contract Research Organizations
KEY PLAYERS
The major key players are 10x Genomics, Dovetail Genomics (Cantata Bio.), S2 Genomics, Inc., NanoString Technologies, Inc., Seven Bridges Genomics, PerkinElmer, Inc., Bio-Techne, Danaher Corporation, Ionpath, Inc., Millennium Science Pty Ltd., and other key players
Key Highlights
Increasing prevalence of cancer and neurological disorders is fueling demand for spatial omics in clinical and translational research.
Technological advancements in imaging and sequencing platforms are enhancing resolution and scalability.
Growing collaborations between academic institutions and biotech firms are accelerating innovation.
Rising demand for personalized medicine and biomarker discovery is expanding the application of spatial omics.
Emergence of AI-driven analytical tools is streamlining data interpretation and boosting efficiency.
Future Scope The future of the spatial omics market lies in the seamless integration of multi-omics data, artificial intelligence, and cloud-based bioinformatics platforms. As spatial technologies evolve to provide higher resolution, throughput, and automation, their adoption in clinical diagnostics is expected to rise significantly. Further, expanding their utility in areas such as regenerative medicine, infectious disease research, and tissue engineering will open new avenues for market growth.
Conclusion The spatial omics market is entering a transformative phase marked by rapid technological advancements and increasing clinical relevance. With its potential to revolutionize disease diagnosis and drug development, spatial omics is emerging as a cornerstone of next-generation biomedical research. Strategic investments and cross-disciplinary collaboration will be key in unlocking its full potential across industries and regions.
Contact Us: Jagney Dave - Vice President of Client Engagement Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK)
Other Related Reports:
Cell Viability Assay Market
Medical Power Supply Market
Post Traumatic Stress Disorder Treatment Market
MRI Guided Neurosurgical Ablation Market
#Spatial Omics Market#Spatial Omics Market Share#Spatial Omics Market Size#Spatial Omics Market Trends#Spatial Omics Market Growth
0 notes
Text
Global Single-Cell Analysis Market to Witness 14% CAGR Due to Increased Use in Stem Cell Research by 2030
The global single-cell analysis Market is expected to witness a growth rate of 14% in the next five years. Continuous innovations in single-cell analysis techniques; rising incidence of cancer and other chronic & infectious diseases; growing shift towards personalized medicine; expansion of the biotechnology and pharmaceutical industries; significant funding and investments for research and development from governments, private companies, and venture capitalists; growing applications in biomedical research; and collaborations between academic institutions, biotechnology companies, and pharmaceutical firms to accelerates the research and development process are some of the key factors driving the single-cell analysis market growth.
Single-cell analysis is a technique used in biological and medical research to study individual cells at the molecular level. Unlike bulk analysis, which examines large cell populations, single-cell analysis reveals cellular heterogeneity by examining differences in gene expression, protein levels, and metabolic activity. It is crucial for understanding disease mechanisms, identifying rare cell populations, and advancing precision medicine. Technologies such as single-cell RNA sequencing (scRNA-seq), flow cytometry, and mass spectrometry enable high-resolution insights into cellular behavior. Applications span cancer research, immunology, neuroscience, and drug development, making single-cell analysis essential for innovations in diagnostics and targeted therapies.
🔗 Want deeper insights? Download the sample report here: https://meditechinsights.com/single-cell-analysis-market/request-sample/
Continuous innovations in single-cell analysis techniques to drive market growth
Innovations in single-cell analysis, including NGS, microfluidics, and mass cytometry, drive market growth by enhancing precision, sensitivity, and throughput. These technologies uncover cellular heterogeneity and rare cell populations, improving diagnostics and personalized medicine. Reduced costs and complexity make them more accessible, expanding their use in oncology, immunology, and stem cell research. Growing applications attract investment and collaborations across academia, biotech, and pharma, fueling market expansion. As advancements continue, single-cell analysis accelerates scientific discovery, leading to breakthroughs in disease research and therapeutic development, reinforcing its critical role in the future of biomedical science.
incidence of cancer and other chronic & infectious diseases to fuel market growth
The rising incidence of cancer and infectious diseases fuels demand for single-cell analysis, enhancing diagnostics and targeted therapies. According to the WHO in 2022, there were an estimated 20 million new cancer cases and 9.7 million deaths, and the cancer cases are expected to increase significantly due to aging populations, lifestyle changes, and environmental factors. Single-cell analysis uncovers tumor heterogeneity, identifying resistant cancer subpopulations and improving personalized medicine. It also aids infectious disease research by analyzing host-pathogen interactions, leading to better treatments and vaccines. Increased investment and innovation drive adoption by pharmaceutical, biotech, and academic institutions, expanding the market as precision medicine and advanced research become essential in disease management and therapeutic development.
Competitive Analysis
The global single-cell analysis market is marked by the presence of established and emerging market players such as Becton, Dickinson and Company; Danaher Corporation; Thermo Fisher Scientific, Inc.; Merck KGaA; Qiagen; 10x Genomics, Inc.; Standard BioTools Inc.; Promega Corporation; Illumina, Inc.; and Bio-Rad Laboratories, Inc.; among others. Some of the key strategies adopted by market players include new product development, strategic partnerships and collaborations, and geographic expansion.
Get PDF Report for Competitive Analysis: https://meditechinsights.com/single-cell-analysis-market/request-sample/
Market Segmentation
This report by Medi-Tech Insights provides the size of the global single-cell analysis market at the regional- and country-level from 2023 to 2030. The report further segments the market based on product, cell type, technique, application, end user.
Market Size & Forecast (2023-2030), By Product, USD Million
Consumables
Beads
Microplates
Reagents
Assay kits
Immunoassays
Cell-based assays
Other Consumables
Instruments
Flow Cytometers
NGS Systems
PCR Instruments
Spectrophotometers
Cell Counters
Microscopes
HCS Systems
Microarrays
Other Instruments
Market Size & Forecast (2023-2030), By Cell Type, USD Million
Human Cell
Animal Cell
Microbial Cell
Market Size & Forecast (2023-2030), By Technique, USD Million
Flow Cytometry
Next-generation Sequencing
Polymerase Chain Reaction
Microscopy
Mass Spectrometry
Others
Market Size & Forecast (2023-2030), By Application, USD Million
Research Applications
Cancer Research
Immunology Research
Neurology Research
Stem Cell Research
Other Research Applications
Medical Applications
Non-invasive Prenatal Diagnosis
In Vitro Fertilization
Circulating Tumor Cell Detection
Other Medical Applications
Market Size & Forecast (2023-2030), By End User, USD Million
Biotechnology & Pharmaceutical Companies
Academic & Research Laboratories
Hospitals & Diagnostic Laboratories
Cell banks & IVF Centers
Other End Users
Others
Market Size & Forecast (2023-2030), By Region, USD Million
North America
US
Canada
Europe
UK
Germany
France
Italy
Spain
Rest of Europe
Asia Pacific
China
India
Japan
Rest of Asia Pacific
Latin America
Middle East & Africa
About Medi-Tech Insights
Medi-Tech Insights is a healthcare-focused business research & insights firm. Our clients include Fortune 500 companies, blue-chip investors & hyper-growth start-ups. We have completed 100+ projects in Digital Health, Healthcare IT, Medical Technology, Medical Devices & Pharma Services in the areas of market assessments, due diligence, competitive intelligence, market sizing and forecasting, pricing analysis & go-to-market strategy. Our methodology includes rigorous secondary research combined with deep-dive interviews with industry-leading CXO, VPs, and key demand/supply side decision-makers.
Contact:
Ruta Halde Associate, Medi-Tech Insights +32 498 86 80 79 [email protected]
0 notes
Text
Unlocking Insights with Omics Data Solutions
In today’s era of precision medicine and advanced biological research, the sheer volume of data generated from various high-throughput technologies demands expert interpretation. Among the most powerful analytical tools are proteomics and multi-omics platforms, which provide researchers with the ability to decode biological systems at an unprecedented depth. Through Protemics data analysis services and multi-omics data integration services, research institutions and biotech companies can harness the full potential of their datasets to drive breakthroughs in healthcare, agriculture, and beyond.

The Role of Proteomics in Biomedical Research
Proteomics refers to the large-scale study of proteins, the fundamental molecules responsible for structure, function, and regulation within organisms. Understanding the proteome—the entire set of proteins expressed by a genome—can offer insights into disease mechanisms, drug responses, and cellular processes. However, raw proteomics data obtained from mass spectrometry or other platforms is complex and requires sophisticated computational tools for analysis.
This is where professional proteomics data analysis services become invaluable. These services employ bioinformatics pipelines, machine learning algorithms, and statistical modeling to identify differentially expressed proteins, annotate biological functions, and map protein-protein interactions. With the help of such services, researchers can accelerate biomarker discovery, validate therapeutic targets, and uncover hidden biological patterns that would otherwise remain elusive.
Integrating Data Across Omics Layers
While proteomics is essential, it represents just one layer of the biological puzzle. Genomics, transcriptomics, metabolomics, and epigenomics each offer unique insights into how living systems operate. To get a complete understanding, it is crucial to integrate these diverse data streams. This is the core principle of Multi omics data integration services.
Multi-omics approaches combine data from multiple omics platforms to build a holistic picture of biological function. This comprehensive analysis enables a deeper understanding of disease progression, gene regulation, and personalized medicine. By merging transcriptomic and proteomic data, for example, researchers can correlate gene expression with protein abundance, gaining more reliable insights than either dataset could provide on its own.
Advanced multi-omics data integration services utilize robust computational frameworks to align, normalize, and analyze heterogeneous datasets. They can reveal regulatory networks, uncover metabolic pathways, and facilitate systems biology studies that are critical for both academic research and clinical applications.
Applications Across Research and Industry
Proteomics and multi-omics data analysis are not confined to laboratories. These services have found applications in pharmaceutical development, clinical diagnostics, agricultural genomics, and environmental studies. In drug discovery, for instance, integrated omics analysis helps identify molecular signatures of diseases and predict drug responses. In agriculture, these approaches support crop improvement and stress resistance studies by uncovering gene-protein-environment interactions.
Moreover, the rise of personalized medicine depends heavily on multi-omics integration. Each patient’s genomic, proteomic, and metabolic profile is unique, and integrating these layers is vital for tailoring treatments and predicting outcomes more accurately.
Challenges and the Need for Expertise
Despite the advantages, integrating and analyzing omics data is not without challenges. The datasets are massive, diverse, and often noisy. Standard analytical tools may fall short in addressing the complexity, necessitating domain-specific expertise and high-end computational infrastructure.
Professional bioinformatics providers that offer specialized proteomics data analysis services and multi-omics data integration services bring both the technical know-how and the computational resources needed to manage such tasks effectively. These services ensure data quality control, statistical rigor, and biological relevance, empowering researchers to derive actionable insights with confidence.
Future Directions in Omics Data Analytics
The field of omics is rapidly evolving. As sequencing and proteomics technologies become more accessible, the demand for integrated, accurate, and scalable data analysis solutions will continue to grow. Emerging fields like single-cell omics and spatial transcriptomics will further increase the need for advanced integration strategies.
To remain at the forefront, researchers and institutions must partner with bioinformatics experts who can offer tailored solutions for their specific datasets and goals. Whether it's through cloud-based platforms, AI-powered analytics, or customized workflows, the future of biological discovery hinges on effective data interpretation.
In conclusion, proteomics and multi-omics integration are revolutionizing the way we understand biology. Organizations looking to maximize the impact of their research should consider expert support from services like those offered by ambioinformatics.com, which specialize in transforming complex omics data into meaningful biological knowledge.
0 notes
Text
Brain Injury and Damage Introduction Brain damage can present itself during the postnatal stage, perinatal, or even the prenatal stage. The prenatal phase arises before birth, and brain damage during this stage harms the brain's development in several ways. It alters cell maturation, proliferation, or migration leading to a future brain malfunction. The perinatal phase entails the phase at the time of birth, while postnatal is the period after (Wilson, 2013). This paper examines psychological, clinical, and biological factors surrounding brain recovery after damage and the underlying recovery factors. Further, it describes numerous clinical interventions essential in the restoration and rejuvenation of compromised brain capabilities. Recovery of Lost Function after Traumatic Brain Injury Neuroplasticity The central nervous system (CNS) assumes innovative roles and potential that encourage secondary recovery mechanisms. By definition, neuroplasticity shapes recovery by running neuronal circuits by employing adaptive transformations to functional and structural levels. These changes take different forms that range from synaptic, molecular, and cellular adjustments to global network changes. Traditionally, the adult brain was believed to be static with neuroplasticity only present during cortical advancements. Today, the belief has a different perspective and proof whereby neuroplasticity after an injury takes a three-stage sequence. Injury leads to cells' death, followed by a reduction in the number of repressive cortical pathways. This happens between one or two days as the secondary neuronal networks get recruited. Later, the activities in the pathways transform to excitatory from the inhibitory state. What follows is the proliferation of the neurons and synaptogenesis. Nonneuronal cells, such as endothelial progenitors, inflammatory cells, and glial cells, together with neuronal cells, replace the destroyed cells, rejuvenate gliotic cells, and revascularize. Few weeks into the injury phase, both axonal sprouting and synaptic markers are upregulated. This process allows cortical changes and remodeling for recovery purposes. Chronic transformations have been part of various studies and have revealed that the healing outcomes vary with age. Preliminary studies have also suggested long-term morphologic adjustments in the hippocampus precisely after TBI. Common changes include neurons recruitment and cell soma growth (Su, Veeravagu & Grant, 2016). Factors Important in Successful Brain Function Recovery Outcomes Various factors influence the recovery process from traumatic brain injury (TBI). Common influencers include injury severity, genetics, treatment response, medical complications, environment, and other underlying conditions. Persons with sustained TBI could exhibit some broad cognitive backgrounds, preexisting health conditions, or other diverse body conditions that could moderate injury effects. When solely or acting collectively and in line with TBI heterogeneity, these attributes can cause changes in the treatment response. Multiple factors can affect the recovery rates of patients after TBI. Such factors include depressions, anxiety disorders, PTSD, social support, post-deployment, and deployment factors and disabilities (Institute of Medicine, 2011). Clinical Interventions Brain injury treatment does not have a predictable and clinical pathway to define physical recovery and surgical excision. This is unlike many other conditions that are friendly in their monitoring phases. To assess brain treatment, patients have to undergo multiple therapies for rehabilitation. For TBI, family interventions are vital because the condition has adverse effects on them, and they also play an essential support role. The interventions are significant in the recovery journey (Gomez-de-Regil, Estrella-Castillo & Vega-Cauich, 2019). Along the improvement journey, therapy sessions are enhanced, and events take longer times from the scale of minutes to several hours. After the patients overcome the acute-care phase, the therapy can go beyond three hours daily. In the case of maladaptive effects such as avoidance of weak limbs, the therapists embrace adaptive behaviors such as the increased effort to use the limb. The therapist's role is to assess the most appropriate pattern that improves both skill creation and its acquisition. On their end, the treatment institutions must embrace tools that promote recovery. The environment should offer learning aids and reinforcement measures. Physicians are expected to apply neuroendocrine and pharmacological methods to aid in developing replacement neurological platforms and assets for cortical reorganization. These should complement speech, physical, cognitive and occupational therapies (Dana Foundation, 2012). Adjuvant therapies aimed at accelerating recovery rates call for advanced characterization to determine the hastened process's effects. Administration of amphetamine has the potential of accelerating recovery too, but for the motor function. Current evidence about non-TBI patients indicates that they need unique stimulus methods to attain neural representation. They require specific network and brain cell assignments. Doctors must assess whether the patients show signs of possible recovery improvements based on the adopted treatment methods. Typical cases include more-intensive versus less intensive patients (Dana Foundation, 2012). Suppose I hold a treatment counselor's position, my specialization in neurobiology about brain injury can be of great use in handling patients. TBI is common but goes highly under-recognized that co-occurs with SUD. In this view, TBI can lead to various behavioral and cognitive consequences that could lead to the patient losing track in adhering to the prescribed treatment. Some TBI patients are absorbed into frug abuse even with diagnosed injuries. Others join undiagnosed, and these scenarios can lead to dire effects. Counselors can predict TBI-affected clients because of the overlapping effects that come with SUD. Other indicators include anxiety, PTSD, and depression. Counselors must be aware of the health status of the client and the effects on their lives. Proper observation can guide the counselors on approaching the clients and seeking the best strategies that fit specific cases such as SUD treatment. They can also engage the clients and provide them with prevention messages and other health interventions that can reduce TBI's recurrence and other brain challenges (Center for Substance Abuse Treatment, 2019). References Center for Substance Abuse Treatment. (2019). Treating clients with Traumatic Brain Injury.Substance Abuse Treatment Advisory,9(2). Retrieved from https://www.brainline.org/article/treating-clients-traumatic-brain-injury Dana Foundation. (2012). Repairing the injured brain. Retrieved from https://dana.org/article/repairing-the-injured-brain/ Gomez-de-Regil, L., Estrella-Castillo, D., & Vega-Cauich, J. (2019). Psychological intervention in Traumatic Brain Injury. Behavioral Neurology. Retrieved from https://doi.org/10.1155/2019/6937832 Institute of Medicine. (2011).Cognitive rehabilitation therapy for Traumatic Brain Injury: evaluating the evidence. Washington, DC: The National Academies Press. https://doi.org/10.17226/13220. Su, Y., Veeravagu, A., & Grant, G. (2016). Neuroplasticity after Traumatic Brain Injury. Taylor & Francis Group. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK326735/ Wilson, J. F. (2013).Biological basis of behavior. Retrieved from https://content.ashford.edu Read the full article
0 notes
Text
god the more I look into it the more parallels I find between chronic fatigue syndrome and autism and it’s wild because like. Extreme heterogeneity in both cases and obviously the diagnostic criteria are a normative attempt to group together a set of behaviors and intolerances to things that the human body “should” be able to tolerate. And also. A lot of it seems to come down to “how does a person/body/brain/mind react when it has a vastly more inefficient system of cellular energy metabolism compared to the idealized Normal Person that the world is built for?” and then ofc how those various reactions get pathologized to hell and back as if it’s simply a matter of not having enough “distress tolerance” and not like “you could mimic this cellular profile by forcing a Healthy Normal Person to not sleep for several days and they’d probably react very similarly to a loud and chaotic airport”
#ME/CFS community 🤝 Autism community#And Many Are Both#I think it’s also funny bc like#all things considered we know a SHIT ton about cellular energy metabolism#but how that translates to brain function overall? No fucking clue#absolute black box#I was talking to my autistic friend the other day about how#I and others around me can see trends in me being more or less ‘autistic’#and it seems to correlate really well with other signs of ‘body can’t make energy right now’#obligatory ‘this user is anti-psychiatry and doesn’t believe that diagnoses are ontologically distinct categories’ tag
1 note
·
View note